half life formula physics
N final N final is the number of remaining radioactive element. Definition of the Half-Life.

Exponential Decay Problem Solving Video Khan Academy
9 rows The isotopic distribution of potassium on the Earth is approximately 93 39 K and 7 41 K.

. The formula for the half-life is obtained by dividing. Hence the half-life of this particular radioactive substance is 3465 years. Go across from the halved.
T 12 0693 03465 2 years. Halve this value and look for this activity. N initial N initial is the number of initial.
The unit of half-life equation for zero order reaction is second 2. For example the half-life of uranium-238 is 45 10 9 years while the half of radium-226 is 1620 years. The half-life formula is commonly used in nuclear physics where it describes the speed at which an atom undergoes radioactive decay.
T12 2 years. T 12 0693 λ. Half-life is the time required for the amount of something to fall to half its initial value.
To calculate the half-life of a sample from a graph. Analyze the table along with the. The mathematical representation of Half life is given by Half life.
Check the original activity where the line crosses the y-axies A0. Half-life formula and unit for first order reaction. The half-life of an atom.
N final N initial 1 2n N final N initial 1 2 n. It is the time requires to decay in half. You mean the half-life of a nucleus I assume.
Since these values are only approximate the total percent abundance of these two isotopes. N0 Initial amount of. When half of the radioactive atom undergoes the decay process the time needed for a quantity to reduce to half of its initial value is the half-life.
The half-life of some radioactive elements is very small for example the half-life of. To see how the number of nuclei declines to half its original value in one half-life let t t 12 in the exponential in the equation N N 0 e λtThis gives N N 0 e λt N 0 e 0693 0500N. Consider a radioactive substance with a mass of 4kg and a.
So suppose a sample has a count rate of 3200 Becquerel Bq at the start what its count rate would be after 8 days would be 116th of. Half Life Formula Physics Spm Each half-life will follow the same general pattern as ceCf-251. The half-life formula used to calculate the first-order reaction is t₁₂.
We have a new and improved read on this topic. The half-life formula is explained and an example problem is worked out. This means a 1 kg radioactive sample today would decay to a 12 kg radioactive sample after 1600 years.
Click Create Assignment to assign this modality to your LMS. The value of the half-life is given by. So if the half-life is two days four half-lives is 8 days.
The half-life of Radium-226 is 1600 years. Naturally a full answer to this question gets very complex taking up much of a semester course in nuclear.

Radio Activity And Half Life Period Iit Jee And Neet Physics

Ionactive Physical Biological And Effective Half Life

Topic 7 Atomic Nuclear And Particle Physics Ib Physics
Radioactive Decay Decay Constant Activity Half Life

5 Ways To Calculate Half Life Wikihow
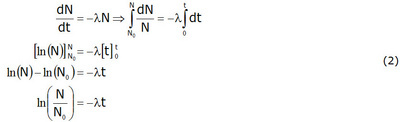
Ionactive Physical Biological And Effective Half Life

Calculation Of Half Life Of Radioactive Substances Half Life O Levels Life

Half Life Spm Physics Form 4 Form 5 Revision Notes

Radioactive Decay Formula Types Examples What Is Radioactive Decay Video Lesson Transcript Study Com
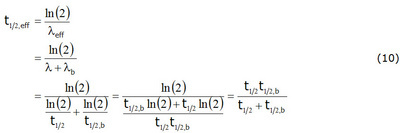
Ionactive Physical Biological And Effective Half Life

12 Half Life Ideas Half Life Chemistry Physical Science

5 Ways To Calculate Half Life Wikihow

An Easy Equation To Calculate The Half Life Of An Isotope Chemistry Physics Youtube



